Welcome to our new newsletter. We know it’s been a while since you’ve heard from us. We hope you’ll enjoy this new publication. Please feel free to share with others and encourage them to subscribe!
This newsletter is for you and we’d like to hear from you! Please send us your AR INBRE news – new publication? presenting somewhere? learned something (i.e. tip/trick) you’d like to share? upcoming event? want to be featured in a spotlight (see below!)? — send it to us!
FOAs Released for Opportunities starting May 1, 2025
In anticipation of a successful renewal of the NIH-supported Arkansas INBRE, we plan to offer two funding opportunities to support PUI faculty-lead research. Both awards would begin support May 1, 2025. The proposal submission deadline is 5 pm, Sept 17, 2024.
- Research Projects (RP) funding is available up to two years with a direct cost budget not to exceed $125,000 per year. Project Leaders will be required to commit 6-person months (50% annual effort) to the project during the award period with Letters of Support from Department Chairs or higher administration officials if needed. Project Leaders will be required to include a minimum of two (2) undergraduate students in the project. The Developmental Research Project Program (DRPP) anticipates awarding eight (8) RPs to start May 2025 with a second round of funding to begin May 2027.
- Pilot Project (PP) funding is for up to 12 months with a direct cost budget not to exceed $50,000. Project Leaders will be required to commit 3-person months (25% annual effort) to the project during the award period with Letters of Support from Department Chairs or upper administration officials if needed. Project Leaders will be required to include a minimum of one (1) undergraduate student in the project. The DRPP anticipates awarding six (6) PPs to start May 2025. The DRPP anticipates annual PP funding opportunities to begin May 1 of subsequent years.
Learn more

New Data Science Capabilities in Arkansas
A grant supplement was awarded to AR INBRE to enhance data science capabilities in the Arkansas. As part of this mechanism, the Bioinformatics Core has developed both hardware and software resources for education and research purposes.
Opportunities include: Cloud computing, Training modules (NIGMS Sandbox), BigOmics Analytics and a new Help Desk for Data Science-related questions. More

Like. Follow. Tag. Share!
Arkansas INBRE’s social media is up and running. Help us build following by liking/following us on these platforms:
Facebook: Arkansas INBRE
Instagram: arkansasinbre (new!)
LinkedIn: arinbre (new page!)
X/Twitter: weARinbre (new profile!)
YouTube: arkansasinbre
Want to see us on another platform? Let us know (email)!

Website Under Construction
Pardon our dust! We are in the process of renovating our website – removing outdated information, updating existing and adding new items. Please bookmark our page and check back to see our progress!
Check out our new 2024 Summer Students Spotlights page (in progress – more spotlights to come)! We would like to do more from across our network. Anyone doing AR INBRE-funded research is invited to send us a short video and blurb on your research!

Save the Dates:
Nov 8-9, Fayetteville
The Arkansas INBRE Research Conference involves participation from colleges and universities in Arkansas and surrounding states in biological sciences, physics and chemistry and biochemistry. The Conference includes talks, presentations and workshops. Registration is now open. More
To receive regular email updates about the Arkansas INBRE research conference, please send a request with your email address to inbreinf@uark.edu.
AR INBRE at NISBRE 2024



Several AR INBRE participants traveled to Washington, DC in mid-June to the 2024 biennial National IDeA Symposium of Biomedical Research Excellence (NISBRE).
- Our PI/Program Director, Dr. Lawrence Cornett, was the co-chair of the “Artificial Intelligence” session.
- Dr. Stephanie Byrum, Data Science Core Director, was a panelist in the “Leveraging the NIGMS Sandbox to Advance Biomedical Research Using Cloud Computing” session.
- Dr. Alan Tackett, Research Technology Core Director, was a panelist in the “Planning for Translational Impact in IDeA Programs” and the “Harnessing Shared Resources: National and Regional Resources for Structural Biology and Biomolecular Analysis” sessions.
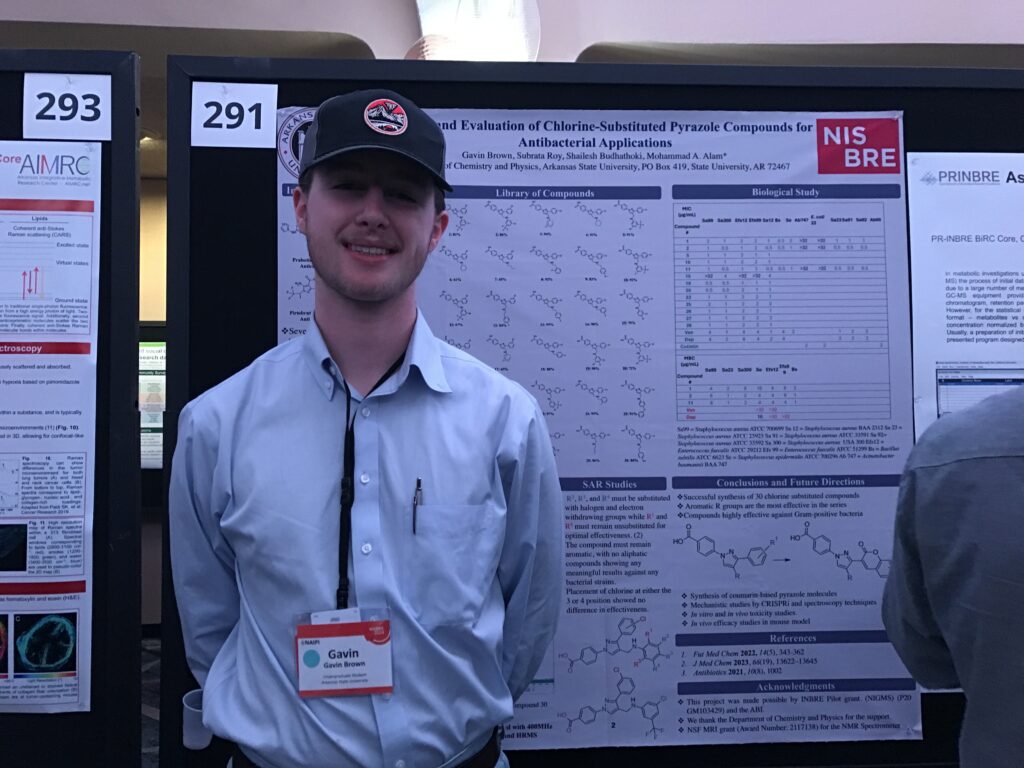
- Student Gavin Brown from Arkansas State University presented poster #291, “Synthesis of novel pyrazole containing compounds as potent antibacterial agents.” Gavin also received a Student Travel Award from the National Association of IDeA Principle Investigators (NAIPI).
- The IDeA National Resource for Quantitative Proteomics (Dr. Tackett, Co-Director) was a NISBRE Diamond Sponsor.
- Additional attendees included our Program Coordinator Dr. Jerry Ware and our INBRE Liaison for PUI Faculty, Dr. Thomas Kelly, and others.
FASEB Webinar: How IDeA Co-Funding Supports Early-Career Investigators. One of the mechanisms by which the NIH IDeA program supports eligible states is co-funding research project grant applications from investigators in IDeA states whose proposals received exemplary scores but fell just shy of the IC’s payline. To demonstrate the powerful role of this component of the IDeA program on research investigators, Eliseo Castillo, PhD, of the University of New Mexico will share how his latest R01 was co-funded by NIGMS on Aug 20, 12 pm CT. More
ABRCMS now accepting abstracts. The 2024 Annual Biomedical Research Conference for Minoritized Scientists (ABRCMS) will take place Nov 13-16 in Pittsburg, PA (Grad Symposium, Nov 16-17). This year, ABRCMS is offering several options for submitting an oral, poster, or ePoster abstract. Abstracts due Sept 6. Travel Awards: Judges due Aug 20; Student and Grad Students due by Aug 26.
SuRE Resource Center Grantsmanship Bootcamp. Join SRC for a free two-day grantsmanship training for faculty and research administrative staff leading up to the Sept 27 SuRE and SuRE-First application deadline. Presenters will focus on key elements of R-series applications and discuss strategies to help you prepare a competitive application. Bootcamp will take place Aug 20-21, 1 to 4 pm ET and consist of two three-hour sessions plus live Q&A. Day 1 includes: Writing a High-Impact Specific Aims Page, Significance & Innovation for NIH R16 Applications and Writing a Successful NIH Approach Section. Day 2 includes: NIH Data Management & Sharing Policy Updates, Making the Most of Your NIH Biosketch and Institutional Letters of Support. More/Register
SuRE Resource Center Webinar: Let’s Replay That Incident, Aug 22, 3 pm ET. Presenters will review strategies to help mentors and supervisors establish a healthy culture of feedback to improve productivity, outcomes, and team dynamics: Understand and practice the Validate, Challenge, Request (VCR) approach for feedback • Learn and practice the Situation, Behavior, Impact (SBI) model for feedback • Learn strategies for setting and communicating expectations. More/register
Seminar: “Introduction to ClinicalTrials.gov.” The IDeA State Consortium for Clinical Research Resource Center (ISCORE-RC) is hosting its next seminar series on Aug 28, 1 to 2 pm. Session is open to all research personnel. On the RSVP form, select “Seminar Series” and then “August.”
During this session, Tara Riddle, WVCTSI clinical trial compliance senior coordinator, will be providing an introduction to ClinicalTrials.gov. Topics to be covered include understanding definitions and timelines, updates on the modernization efforts by the NIH, responsibilities of registration, and noncompliance pitfalls and penalties. Continuing education credits will be offered for this presentation.
NIH Center for Scientific Review Open Position: Director, Division of Receipt and Referral. The Director of the Division of Receipt and Referral (DRR) serves as an important advisor to the Director, Center for Scientific Review (CSR). The DRR Director supervises at least 10 Federal employees and coordinates the work of seven contract staff. Apply by Aug 29. More
NIH High-Risk, High-Reward Research (HRHR). The program’s four awards provide a diverse set of funding opportunities:
NIH Director’s New Innovator Award for early career investigators who are within 10 years of their final degree or post-grad clinical training and have not yet received a research project grant or equivalent NIH grant. Due Aug 19.
NIH Director’s Transformative Research Award is open to individuals and teams of investigators who propose unconventional research that could create or challenge existing paradigms. Due Sept 3.
NIH Director’s Early Independence Award is for junior scientists who have recently received their doctoral degree or completed their post-grad clinical training to skip traditional postdoc training and move immediately into independent research positions. Due Sept 6.
NIH Director’s Pioneer Award for investigators at all career levels to pursue new research directions and develop groundbreaking, high-impact approaches within broad areas of biomedical, behavioral or social science. Due Sept 9.
Aug 24: LOI: NIH CCRP Initiative: Chem Threat Agent-induced Pulmonary & Ocular Pathophysio Mechanisms (R01)
Aug 26: NIH NIMH Dev AIDS Res Ctr on Mental Health & HIV/AIDS (P30)
Aug 27: 2 pm, NCFDD Webinar LIVE ON ZOOM: Navigating the Academic Job Market: Practical Tips & Strategies
Aug 30: NIIH Lasker Clin Res Scholars Prog (Si2/R00)
Sept 2: Arkansas INBRE Program Office will be closed for Labor Day
Sept 4
— 2025 Amer Heart Assoc Predoc Fellow
— LOI: NIH NINDS/NIA Neuropath Interactions Between COVID-19 & ADRD (R01)
— LOI: NIH NINDS/NIA Interaction Between Environmental Factors & Lewy Body Dementia (R01)
Sept 5
— 2025 Amer Heart Assoc Postdoc Fellow
— NIH NIGMS Interactive Digital Media (IDM) Biomedical Science Resources for Pre-College Students and Teachers (SBIR) (R43/R44)
— LOI: NIH NCI Cancer Prevent & Control Clin Trials Grant Prog (R01)
— LOI: NIH NCI Impacts of climate change across the cancer control continuum (R01)
PHS 2024-2 Omnibus Solicitation:
— of the NIH and CDC for SBIR Grant Apps (R43/R44)
— of the NIH, CDC and FDA for SBIR Grant Apps (R43/R44] Clin Trial Not Allw’d)
— of the NIH for STTR Grant Apps (R41/R42 Clin Trial Not Allw’d)
— of the NIH for STTR Grant Apps (R41/R42] Clin Trial Req’d)
Sept 8: LOI: NIH NCI Exploratory Grants in Cancer Control (R21)
Sept 10
— LOI: NIH NIA/NCCIH Safety & Early Efficacy Studies of Psychedelic-Assisted Therapy for Chronic Pain in Older Adults (UG3/UH3)
— NIH Time-Sensitive Obesity Policy and Program Evaluation (R01)